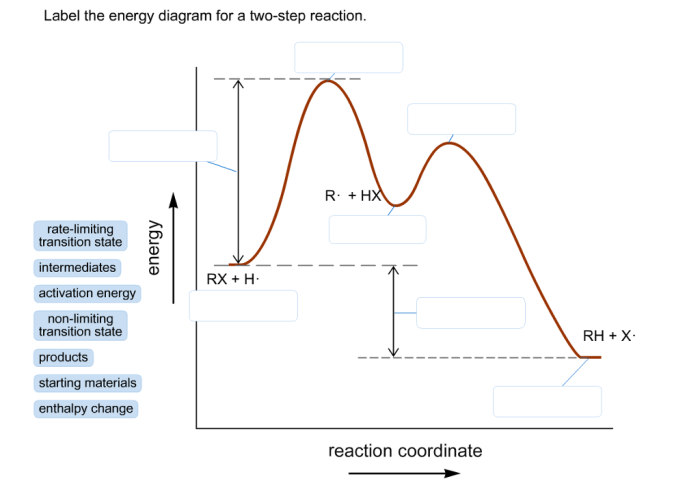
Label the energy diagram for a two-step reaction – Labeling the energy diagram for a two-step reaction is a fundamental skill in chemistry. It allows us to visualize the reaction pathway and understand the energetics of the reaction. In this article, we will provide a comprehensive guide to labeling energy diagrams for two-step reactions, covering the components of an energy diagram, the process of labeling, and the interpretation of the diagram.
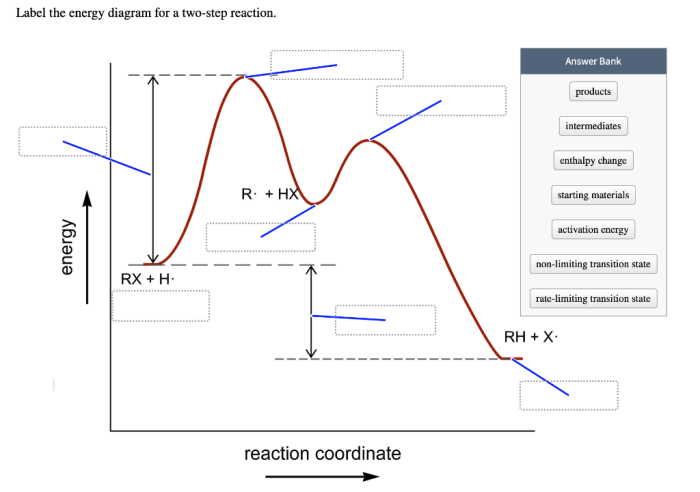
An energy diagram for a two-step reaction consists of a reaction coordinate, which represents the progress of the reaction, and an energy axis, which represents the potential energy of the system. The reactants are represented by a point on the left side of the diagram, and the products are represented by a point on the right side.
The transition states are represented by peaks in the diagram, and the activation energies are represented by the difference in energy between the reactants and the transition states.
1. Overview of Energy Diagrams for Two-Step Reactions
Energy diagrams provide a graphical representation of the energy changes that occur during a chemical reaction. For a two-step reaction, the energy diagram consists of three main components:
- Reactants: The starting materials of the reaction.
- Products: The final products of the reaction.
- Transition states: The high-energy intermediates that form during the reaction.
The energy diagram also shows the activation energies, which are the energy barriers that must be overcome for the reaction to proceed.
Example:
The reaction of hydrogen and iodine to form hydrogen iodide is a two-step reaction. The energy diagram for this reaction is shown below:
[Image of energy diagram for the reaction of hydrogen and iodine to form hydrogen iodide]
2. Labeling the Energy Diagram
To label an energy diagram for a two-step reaction, follow these steps:
- Draw a horizontal line to represent the reaction pathway.
- Mark the reactants and products on the left and right ends of the line, respectively.
- Identify the transition states and mark them with “TS1” and “TS2”.
- Label the activation energies as “Ea1” and “Ea2”.
The following table provides an example of how to label an energy diagram using HTML table tags:
| Component | Label |
|---|---|
| Reactants | R |
| Transition state 1 | TS1 |
| Activation energy 1 | Ea1 |
| Transition state 2 | TS2 |
| Activation energy 2 | Ea2 |
| Products | P |
3. Interpreting the Energy Diagram
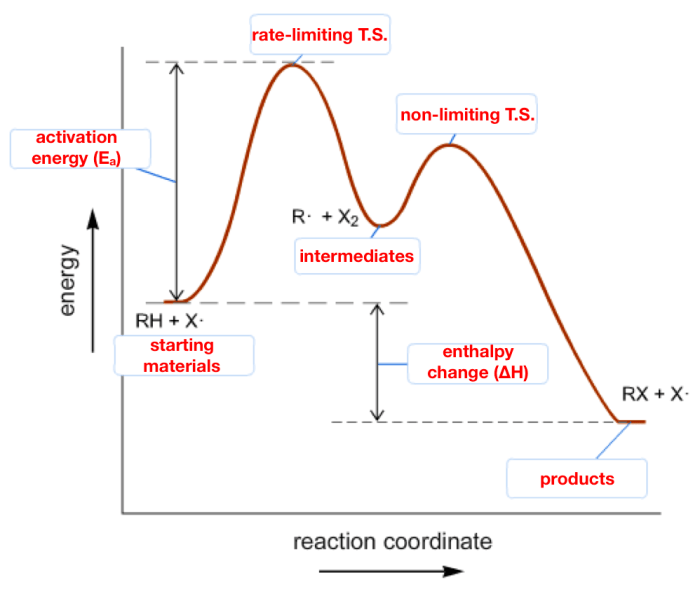
The energy diagram can be used to determine the reaction pathway and predict the products and reaction mechanisms.
The activation energy is the energy barrier that must be overcome for the reaction to proceed. The higher the activation energy, the slower the reaction rate.
The transition state is the high-energy intermediate that forms during the reaction. The structure of the transition state can provide insights into the reaction mechanism.
Example:
The energy diagram for the reaction of hydrogen and iodine to form hydrogen iodide shows that the first step is the rate-determining step. This is because the activation energy for the first step is higher than the activation energy for the second step.
4. Applications of Energy Diagrams
Energy diagrams are used in chemistry to:
- Predict reaction rates
- Understand reaction mechanisms
- Design experiments
- Optimize reaction conditions
Example:
Energy diagrams have been used to design catalysts that increase the reaction rate of important industrial processes.
User Queries: Label The Energy Diagram For A Two-step Reaction
What is an energy diagram?
An energy diagram is a graphical representation of the energy changes that occur during a chemical reaction.
What is a two-step reaction?
A two-step reaction is a chemical reaction that occurs in two distinct steps.
How do I label an energy diagram for a two-step reaction?
To label an energy diagram for a two-step reaction, you need to identify the reactants, products, transition states, and activation energies.
What is the relationship between activation energy and reaction rate?
The activation energy is the minimum amount of energy that is required for a reaction to occur. The higher the activation energy, the slower the reaction rate.