Embarking on a journey into the realm of chemistry, the mixtures elements and compounds worksheet serves as an invaluable tool for understanding the fundamental building blocks of matter. Delving into the intricacies of mixtures, elements, and compounds, this worksheet unveils the distinctions between these entities and provides a roadmap for exploring their properties and behaviors.
As we traverse this educational landscape, we will encounter various types of mixtures, delve into the methods employed to separate them, and unravel the mysteries of chemical reactions. Through stoichiometry, we will decipher the quantitative relationships within chemical equations, while percent yield calculations will shed light on the efficiency of chemical processes.
Mixtures, Elements, and Compounds
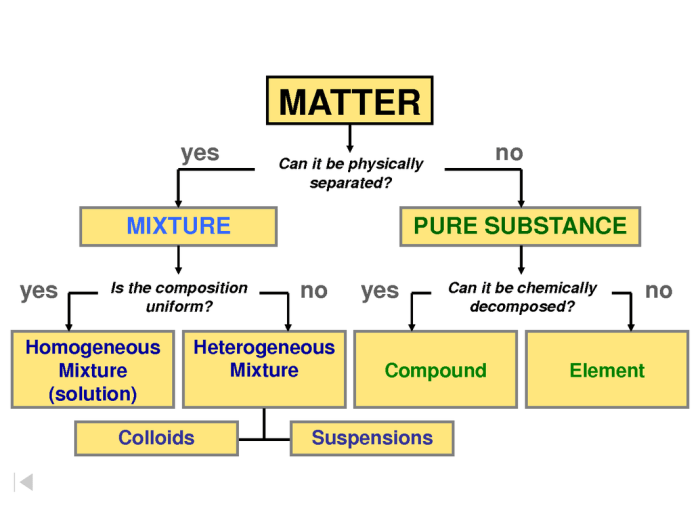
Mixtures, elements, and compounds are the basic building blocks of matter. Mixtures are combinations of two or more substances that are not chemically bonded together. Elements are pure substances that cannot be broken down into simpler substances by chemical means.
Compounds are pure substances that are made up of two or more elements that are chemically bonded together.
Mixtures can be homogeneous or heterogeneous. Homogeneous mixtures are mixtures in which the components are evenly distributed throughout the mixture. Heterogeneous mixtures are mixtures in which the components are not evenly distributed throughout the mixture.
Compounds are always homogeneous mixtures. Elements are always pure substances.
Examples of Mixtures and Compounds, Mixtures elements and compounds worksheet
- Air is a homogeneous mixture of water and air.
- Salt water is a homogeneous mixture of water and salt.
- Sand is a heterogeneous mixture of sand and other minerals.
- Gold is an element.
- Water is a compound.
FAQs: Mixtures Elements And Compounds Worksheet
What is the difference between a mixture and a compound?
A mixture is a combination of two or more substances that retain their individual chemical identities, while a compound is a new substance formed when atoms of different elements combine chemically.
How can we separate a heterogeneous mixture?
Heterogeneous mixtures can be separated using methods such as filtration, distillation, or chromatography, which rely on differences in physical properties like particle size or solubility.
What is the purpose of a chemical equation?
A chemical equation provides a symbolic representation of a chemical reaction, showing the reactants, products, and their stoichiometric proportions.