Periodic trends atomic radius answer key unlocks the secrets of atomic structure, revealing the fascinating patterns that govern the size of atoms. This guide delves into the concept of atomic radius, exploring its variations across the periodic table and the underlying factors that shape these trends.
Embark on a journey through the periodic table, unraveling the mysteries of atomic radius and its profound implications in chemistry and beyond.
Atomic Radius
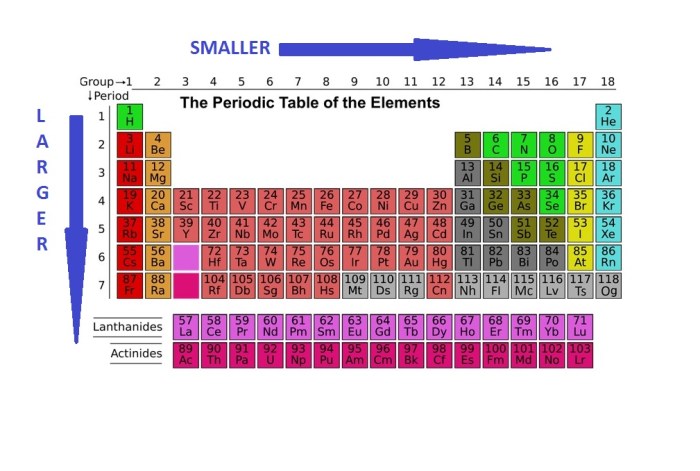
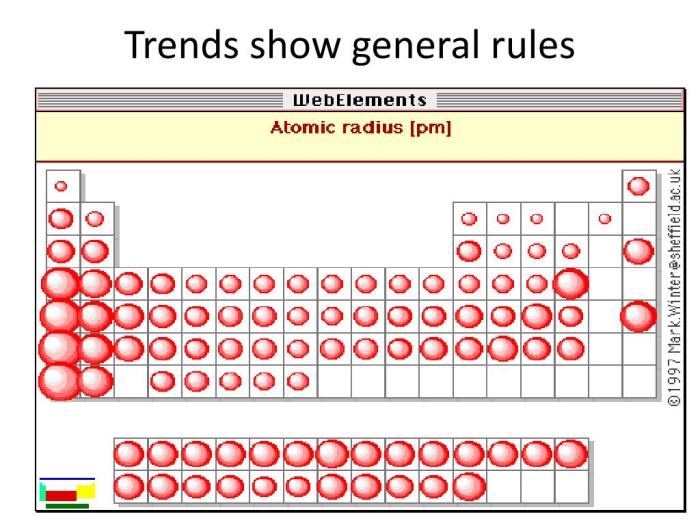
Atomic radius is a measure of the size of an atom. It is defined as the distance from the nucleus to the outermost electron shell. The atomic radius of an element can be measured in picometers (pm), which are one trillionth of a meter (10^-12 m).
The atomic radius of an element is not a fixed value, but rather depends on the number of electrons in the atom and the number of protons in the nucleus. In general, the atomic radius decreases as the number of electrons increases and the number of protons decreases.
Periodic Trends in Atomic Radius, Periodic trends atomic radius answer key
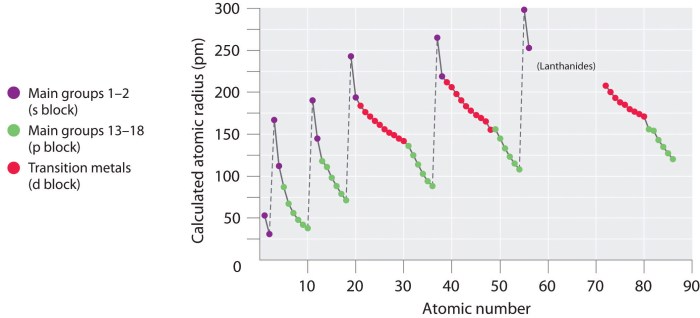
The atomic radius of an element shows a periodic trend across the periodic table. In general, the atomic radius decreases from left to right across a period (row) and increases from top to bottom within a group (column).
The decrease in atomic radius across a period is due to the increase in the number of protons in the nucleus. As the number of protons increases, the electrostatic attraction between the nucleus and the electrons increases, pulling the electrons closer to the nucleus and reducing the atomic radius.
The increase in atomic radius down a group is due to the increase in the number of electron shells. As the number of electron shells increases, the outermost electrons are further away from the nucleus, resulting in a larger atomic radius.
Factors Affecting Atomic Radius
- Number of electrons:The number of electrons in an atom has a significant impact on its atomic radius. In general, the more electrons an atom has, the larger its atomic radius.
- Number of protons:The number of protons in an atom also affects its atomic radius. In general, the more protons an atom has, the smaller its atomic radius.
- Shielding effect:The shielding effect refers to the ability of inner electrons to block the attraction between the nucleus and the outermost electrons. The more inner electrons an atom has, the greater the shielding effect, and the larger the atomic radius.
Applications of Atomic Radius
The atomic radius of an element is a useful property that can be used to predict the properties and behaviors of elements and compounds. For example, the atomic radius can be used to:
- Predict the chemical reactivity of an element
- Determine the type of chemical bond that an element will form
- Calculate the density of a compound
- Predict the melting point and boiling point of a compound
Question & Answer Hub: Periodic Trends Atomic Radius Answer Key
What is atomic radius?
Atomic radius is a measure of the distance from the nucleus to the outermost electron shell of an atom.
How does atomic radius vary across the periodic table?
Atomic radius generally increases down a group and decreases across a period from left to right.
What factors influence atomic radius?
Atomic radius is influenced by the number of electron shells, effective nuclear charge, and shielding effect.
What are the applications of atomic radius?
Atomic radius is used to predict properties such as melting point, boiling point, and chemical reactivity.