Embark on an enriching journey with AP Chemistry Unit 3 Notes, where the intricate world of thermodynamics, equilibrium, acids and bases, solutions, electrochemistry, kinetics, and nuclear chemistry unfolds before your eyes. This comprehensive guide promises to ignite your curiosity and empower you with a profound understanding of the subject.
As you delve into these notes, prepare to unravel the mysteries of entropy, explore the dynamics of equilibrium, and delve into the fascinating realm of electrochemistry. With clear explanations, engaging examples, and a conversational tone, this resource will transform your learning experience into an enthralling adventure.
Thermodynamics
Thermodynamics is a branch of physical chemistry that studies the relationships between heat, work, and the properties of matter.
The laws of thermodynamics are fundamental principles that govern these relationships. The first law of thermodynamics states that energy cannot be created or destroyed, only transferred or transformed. The second law of thermodynamics states that the entropy of an isolated system always increases over time.
The third law of thermodynamics states that the entropy of a perfect crystal at absolute zero is zero.
Entropy
Entropy is a measure of the disorder of a system. A system with high entropy is more disordered than a system with low entropy. Entropy always increases over time, which means that the universe is becoming increasingly disordered.
Exothermic and Endothermic Reactions, Ap chemistry unit 3 notes
Exothermic reactions are reactions that release heat. Endothermic reactions are reactions that absorb heat. The enthalpy change of a reaction is the amount of heat that is released or absorbed during the reaction. Enthalpy is a state function, which means that it depends only on the initial and final states of the system, not on the path taken between the two states.
Equilibrium
Chemical equilibrium is a state in which the concentrations of the reactants and products of a chemical reaction do not change over time. This means that the forward and reverse reactions are occurring at the same rate.
There are a number of factors that can affect equilibrium, including temperature, pressure, and the concentration of the reactants and products. For example, increasing the temperature of a reaction will shift the equilibrium to the side of the products, while increasing the pressure will shift the equilibrium to the side of the reactants.
The Equilibrium Constant
The equilibrium constant is a number that describes the relative amounts of reactants and products at equilibrium. The equilibrium constant is a constant for a given reaction at a given temperature.
Keq= [products]/[reactants]
The equilibrium constant can be used to predict the direction of a reaction and to calculate the concentrations of the reactants and products at equilibrium.
Acids and Bases
Acids and bases are two fundamental concepts in chemistry that describe the chemical properties of substances. Acids are substances that donate protons (H+ ions), while bases are substances that accept protons.
Properties of Acids
* Acids have a sour taste.
- Acids react with metals to produce hydrogen gas.
- Acids turn blue litmus paper red.
- Acids are corrosive and can damage skin and other tissues.
- Acids have a pH below 7.
Properties of Bases
* Bases have a bitter taste.
- Bases feel slippery to the touch.
- Bases turn red litmus paper blue.
- Bases can neutralize acids.
- Bases have a pH above 7.
The pH Scale
The pH scale is a measure of the acidity or basicity of a solution. The pH scale ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are basic.
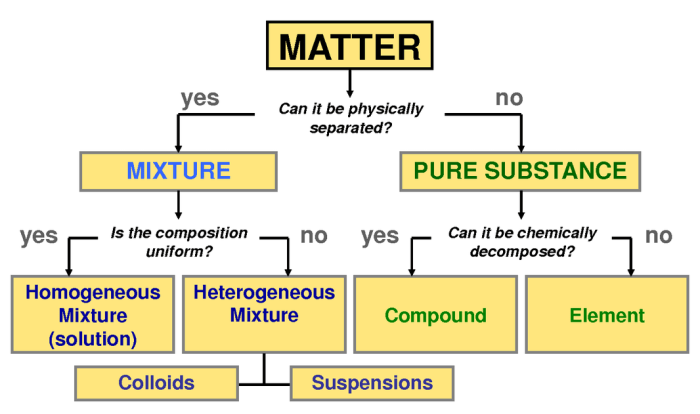
Solutions
A solution is a homogeneous mixture of two or more substances. The substance present in the largest amount is called the solvent, while the other substances are called solutes. Solutions can be classified into different types based on the physical state of the solvent and solute.
Types of Solutions
- Solid solutionsare formed when a solid solute dissolves in a solid solvent. Examples include alloys, such as steel (iron and carbon) and brass (copper and zinc).
- Liquid solutionsare formed when a liquid solute dissolves in a liquid solvent. Examples include saltwater (sodium chloride in water) and sugar water (sucrose in water).
- Gaseous solutionsare formed when a gaseous solute dissolves in a gaseous solvent. Examples include air (nitrogen, oxygen, and other gases in the atmosphere) and helium balloons (helium in air).
Solubility
Solubility is the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature. The solubility of a substance depends on several factors, including the nature of the solute and solvent, temperature, and pressure.
Electrochemistry
Electrochemistry is the branch of chemistry that deals with the relationship between electrical energy and chemical change. It is a fascinating field that has applications in a wide variety of areas, such as batteries, fuel cells, and corrosion.
Electrochemical cells are devices that use chemical reactions to generate electricity or to drive chemical reactions. There are two main types of electrochemical cells: galvanic cells and electrolytic cells.
Galvanic Cells
Galvanic cells are electrochemical cells that generate electricity from a spontaneous chemical reaction. The most common type of galvanic cell is the battery. Batteries consist of two electrodes (a positive electrode and a negative electrode) that are immersed in an electrolyte solution.
The chemical reaction that occurs in the battery generates electrons, which flow through an external circuit, creating an electric current.
If you’re feeling confident with your AP Chemistry Unit 3 notes, why not challenge yourself with an EPA Type 1 practice test ? It’s a great way to test your knowledge and identify areas where you might need to brush up.
Once you’ve completed the practice test, you can revisit your Unit 3 notes to reinforce the concepts you’ve learned.
Electrolytic Cells
Electrolytic cells are electrochemical cells that use electricity to drive a chemical reaction. The most common type of electrolytic cell is the electrolysis cell. Electrolysis cells consist of two electrodes (a positive electrode and a negative electrode) that are immersed in an electrolyte solution.
When an electric current is passed through the cell, the electricity drives the chemical reaction, causing the reactants to be converted into products.
Redox Reactions
Redox reactions are chemical reactions that involve the transfer of electrons between atoms or ions. Redox reactions are important in a wide variety of chemical processes, such as combustion, respiration, and photosynthesis.
In a redox reaction, one species is oxidized (loses electrons) and another species is reduced (gains electrons). The species that is oxidized is called the reducing agent, and the species that is reduced is called the oxidizing agent.
Kinetics
Chemical kinetics is the study of reaction rates, the changes in the concentrations of reactants and products with time. Understanding reaction rates is crucial in various fields, including industrial chemistry, environmental science, and medicine.
Several factors influence reaction rates, including the nature of the reactants, their concentrations, temperature, the presence of a catalyst, and the solvent.
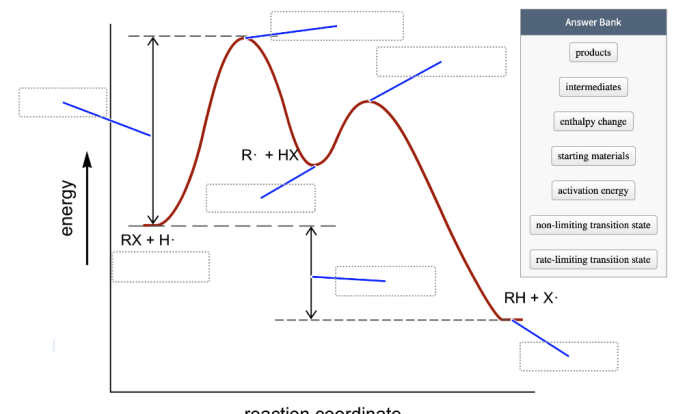
Activation Energy
Activation energy is the minimum energy required for a reaction to occur. It represents the energy barrier that must be overcome for the reactants to transform into products. Reactions with higher activation energies proceed more slowly.
Nuclear Chemistry
Nuclear chemistry deals with the study of the structure, properties, reactions, and applications of atomic nuclei. It involves the study of the fundamental particles that make up the nucleus, such as protons and neutrons, and the forces that hold them together.Nuclear
chemistry has applications in various fields, including nuclear power, medicine, and archaeology. Nuclear power plants use nuclear reactions to generate electricity, while nuclear medicine uses radioactive isotopes for diagnostic and therapeutic purposes. Archaeologists use radioactive isotopes to date ancient artifacts.
Types of Nuclear Reactions
There are several types of nuclear reactions, including:
-
-*Nuclear fission
The splitting of a heavy nucleus into two or more lighter nuclei, releasing a large amount of energy.
-*Nuclear fusion
The combining of two or more light nuclei into a heavier nucleus, also releasing a large amount of energy.
-*Radioactive decay
The spontaneous emission of radiation from an unstable nucleus, resulting in the formation of a more stable nucleus.
Radioactivity
Radioactivity is the emission of radiation from the nucleus of an atom. Radiation can be in the form of alpha particles (helium nuclei), beta particles (electrons or positrons), or gamma rays (high-energy photons).Radioactive isotopes are atoms with an unstable nucleus that undergoes radioactive decay.
The rate of radioactive decay is constant for a given isotope and is measured by its half-life, which is the time it takes for half of the radioactive atoms in a sample to decay.
Questions and Answers: Ap Chemistry Unit 3 Notes
What is the significance of entropy in thermodynamics?
Entropy measures the degree of disorder or randomness within a system. It plays a crucial role in determining the spontaneity and direction of chemical reactions.
How does equilibrium affect chemical reactions?
Equilibrium establishes a dynamic balance between reactants and products, influencing the extent to which a reaction proceeds. Factors such as temperature, pressure, and concentration can shift the equilibrium position.
What is the difference between a strong acid and a weak acid?
Strong acids completely dissociate in water, releasing a large number of hydrogen ions (H+). Weak acids, on the other hand, only partially dissociate, resulting in a lower concentration of H+ ions.